Women with symptoms at mammography screening visit have higher risk of interval breast cancer
Efforts to reduce breast cancer mortality through early detection and diagnosis has intensified in the recent decade. Based on the evidence from the randomized controlled trials and observational studies, women attending mammography screening have about 30–40 % reduced risk of breast cancer mortality.1,2 An important risk factor, ‘breast symptoms’ reported by clinical examination or by women during screening visit, remains overlooked. The single reason is that many population-based mammography-screening programmes have not collected or analyzed information on breast symptoms.
In Finland, after the beginning of mammography screening programme in 1987, collection of information on breast symptoms reported by women at the screening visit has been in practice. Women aged 50–69 years old eligible for screening mammography are invited every two years.3 Information on breast symptoms, such as lump, retraction, or nipple discharge are collected before the mammography is taken. We conducted a study to assess the association between these breast symptoms reported at the screening visit and the risk of interval breast cancer.
Study methods
We performed a matched cohort study based on the follow-up of the ongoing Finnish National Breast Cancer Screening Programme. The study used three registries, the Finnish Cancer Registry (1953–2014), the Mass Screening Registry (1992–2012) and the Central Population Registry (1992–2014) to extract information on the study participants at the individual level. The three exposed groups consisting of three symptom types were frequency matched to the reference group consisting of respective asymptomatic visits by age at the screening visit (within two years), municipality of invitation, year of invitation (2-year band) and number of visits in the past.
Interval cancers were defined as breast cancers diagnosed in screened women before the next screening visit or within a period equal to a screening interval with (i) negative mammography at the index visit (i.e., test negative); (ii) positive mammography at the index visit, but negative further assessment (i.e., episode negative); and (iii) positive further assessment but a date of diagnosis > 6 months after mammography.4 The follow-up time started from the index visit (visit with breast symptom) in 1 January 1992 to 31 December 2012 and ended at the date of emigration or death, upon diagnosis of interval cancer or at the end of follow-up i.e., 31 December 2014, whichever occurred first. The follow-up time for interval cancers started at 1 month and ended at the subsequent screening visit at 23 months. We compared interval breast cancer risk using Cox proportional hazard regression among women with and without reported symptoms at the index screen.
Risk of interval breast cancer
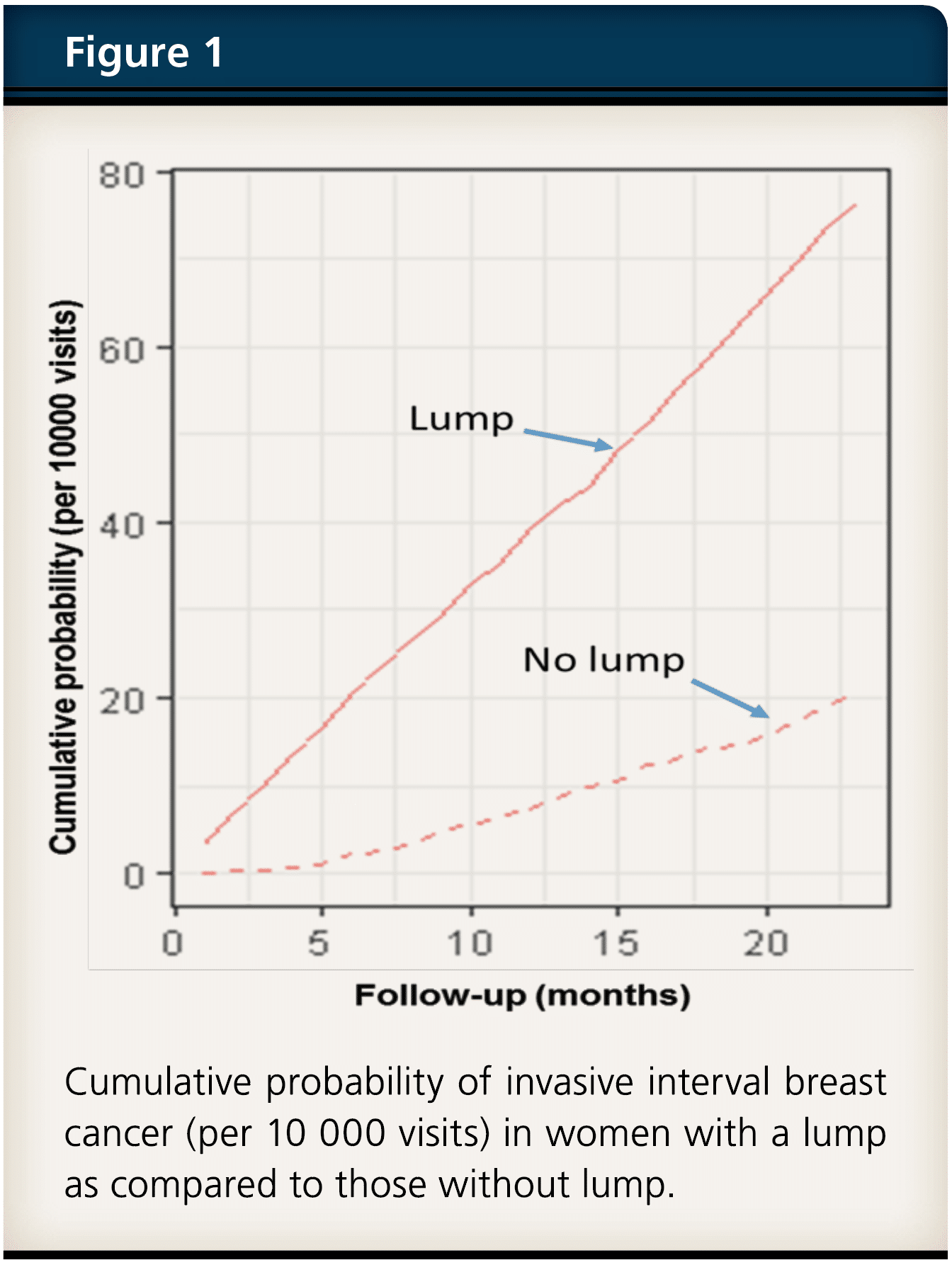
The age-adjusted risk of interval breast cancer was significantly higher for a lump (adjusted hazard ratio 3.7, 95 % CI 3.0–4.6), retraction (adjusted hazard ratio 1.5, 95 % CI 1.1–1.9), and nipple discharge (adjusted hazard ratio 2.4, 95 % CI 1.6–3.7) compared with those without these symptoms. In addition, the risk of non-localized interval breast cancer was also greater for all three symptoms in comparison with visits without symptoms. The incidence of interval cancer after a visit with a lump or nipple discharge increased rather rapidly. Remarkably, in mammography-negative visits with a lump, the cumulative incidence curve detached from the no lump curve immediately after the first month of visit (Figure 1). In absolute terms, per 10 000 women who attended and reported a lump, 70 women were diagnosed with invasive interval breast cancers, i.e., within 24 months compared with about 20 cancers diagnosed without symptoms.
Clinical and public health implication
The higher interval cancer incidence within six months after a negative mammography with a lump is a clear concern for the programme. This indicates that further assessment is needed more frequently and there needs to be highly stringent diagnostic evidence for a decision not to carry out a full further assessment including a core biopsy in these cases. Taking into account the high incidence of advanced interval cancers in symptomatic women, it is likely that protection by biennial screening visit is not sufficient even after potential improvements in further assessments. Hence, we recommend intensified diagnostic service and follow-up with a shorter than the 2-yearly interval for the symptomatic women. For two of the studied symptoms (lump and nipple discharge), the interval cancer incidence increased so rapidly after the screening visit with these symptoms that the first follow-up visit could take place shortly after the visit.
-
Our study provides comprehensive evidence that women with breast symptoms at visits within the population-based breast cancer screening programme have a clearly increased risk of breast cancer. Improvement in the guidelines on screening and clinical services are needed to better serve women with breast symptoms.
-
Conflict of interest: The authors have no conflict of interest or disclosures. All authors declare no financial or other relationships or activities that could appear to have influenced the submitted work.
-
Link to the article: https://rdcu.be/bh1v9
-
1. Lauby-Secretan B, et al. Breast-cancer screening – viewpoint of the IARC working group. N Engl J Med 2015;372:2353–2358. 2. Heinävaara S, Sarkeala T, Anttila A. Impact of organised mammography screening on breast cancer mortality in a case-control and cohort study. British Journal of Cancer 2016;1–7:doi:10.1038/bjc.2016.68. 3. Finnish Cancer Registry. Breast cancer screening; Mass screening programme 2018. Available at http://www.cancer.fi/syoparekisteri/en/mass-screening-registry/breast_cancer_screening/screening_programme/. 4. Houssami N, Irwig L, Ciatto S. Radiological surveillance of interval breast cancers in screening programmes. Lancet Oncol 2006;7:259–265.

